# Unlocking Chemical Reactions: Your Comprehensive Guide to the Chemical Activity Chart
The chemical activity chart is an indispensable tool for anyone studying or working with chemistry. From predicting reaction outcomes to understanding corrosion, this chart provides critical insights into the relative reactivity of different metals and elements. This comprehensive guide will delve into the intricacies of the chemical activity chart, exploring its principles, applications, and limitations. We aim to provide you with a deep understanding of this essential resource, empowering you to use it effectively in your studies and professional endeavors. You’ll learn not just *what* the chemical activity chart is, but *why* it works and *how* to apply it in practical situations. This guide goes beyond simple definitions, offering advanced insights and real-world examples. We will also address common misconceptions and provide expert advice to ensure you master this valuable tool.
## Understanding the Chemical Activity Chart: A Deep Dive
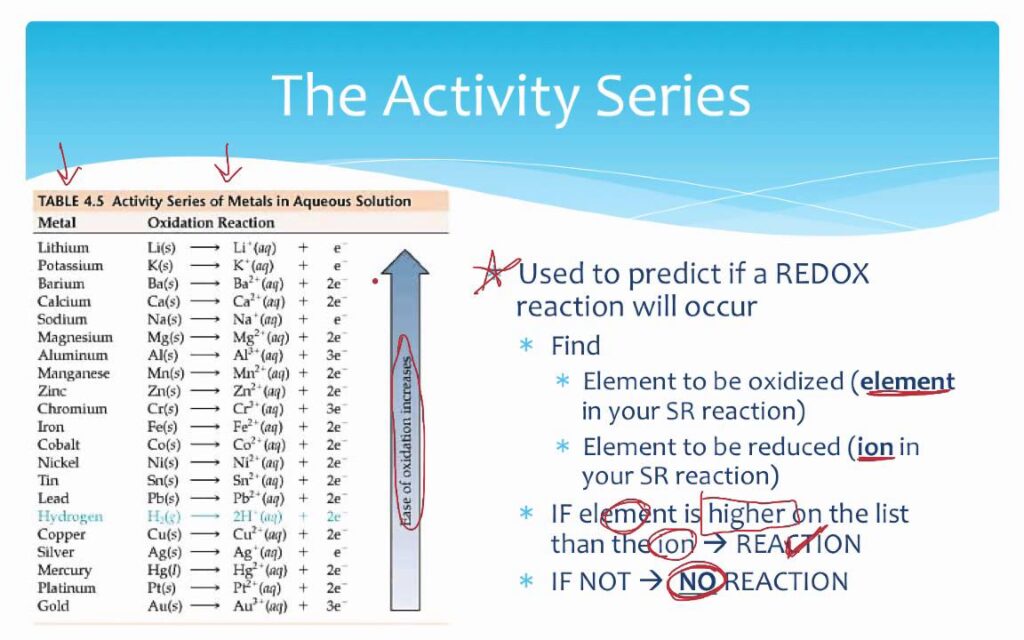
The chemical activity chart, also known as the activity series, ranks metals (and sometimes hydrogen) in order of their decreasing ease of oxidation. In simpler terms, it tells you which metals are more likely to lose electrons and form positive ions in a chemical reaction. This ranking is based on the standard reduction potentials of the elements involved. The chart is typically presented with the most reactive metals at the top and the least reactive at the bottom. Understanding the underlying principles of the chart is crucial for its effective application.
### Core Concepts and Advanced Principles
The foundation of the chemical activity chart lies in the concept of standard reduction potentials. These potentials measure the tendency of a chemical species to be reduced (gain electrons). A higher (more positive) reduction potential indicates a greater tendency to be reduced, while a lower (more negative) reduction potential indicates a greater tendency to be oxidized. The activity series is derived from these standard reduction potentials, with elements having lower reduction potentials positioned higher on the chart.
**Key Principles:**
* **Oxidation and Reduction:** Chemical reactions often involve the transfer of electrons. Oxidation is the loss of electrons, while reduction is the gain of electrons.
* **Redox Reactions:** Reactions involving both oxidation and reduction are called redox reactions.
* **Displacement Reactions:** A more reactive metal can displace a less reactive metal from its compound in a solution. This is the fundamental principle behind using the activity series to predict reaction outcomes.
* **Standard Reduction Potentials:** These values are determined experimentally and provide a quantitative measure of an element’s tendency to be reduced.
**Advanced Considerations:**
* **Non-Standard Conditions:** The activity series is based on standard conditions (25°C, 1 atm pressure, 1 M concentration). Deviations from these conditions can affect the relative reactivity of metals.
* **Overpotential:** In some electrochemical reactions, an overpotential is required to overcome kinetic barriers. This can influence the observed reactivity of metals.
* **Passivation:** Some metals, like aluminum and chromium, form a protective oxide layer on their surface, which can significantly reduce their reactivity.
### Importance and Current Relevance
The chemical activity chart remains a vital tool in various fields, including:
* **Electrochemistry:** Predicting the feasibility of redox reactions in batteries and fuel cells.
* **Corrosion Science:** Understanding and preventing the corrosion of metals in different environments.
* **Metallurgy:** Extracting metals from their ores using displacement reactions.
* **Chemical Synthesis:** Designing and controlling chemical reactions involving metals.
Recent advancements in materials science and electrochemistry have further highlighted the importance of the activity series. For example, the development of new battery technologies relies heavily on understanding the redox behavior of different materials, which is directly informed by the chemical activity chart. Furthermore, the increasing focus on sustainable materials and corrosion-resistant alloys has driven renewed interest in utilizing the activity series to design and optimize these materials.
## Galvanic Cells and the Chemical Activity Chart
One of the most direct applications of the chemical activity chart is in understanding and designing galvanic cells (batteries). A galvanic cell harnesses the spontaneous redox reaction between two different metals to generate electrical energy. The chemical activity chart allows us to predict the voltage and overall feasibility of such a cell.
### Expert Explanation
A galvanic cell consists of two half-cells, each containing a metal electrode immersed in a solution of its ions. The two half-cells are connected by a salt bridge, which allows ions to flow between the solutions and maintain electrical neutrality. The metal higher on the activity series will act as the anode (where oxidation occurs), while the metal lower on the activity series will act as the cathode (where reduction occurs).
The difference in standard reduction potentials between the two metals determines the cell potential (voltage) of the galvanic cell. A larger difference in reduction potentials results in a higher cell potential, indicating a more spontaneous and efficient reaction.
For example, a galvanic cell consisting of a zinc electrode in a zinc sulfate solution and a copper electrode in a copper sulfate solution will have a positive cell potential because zinc is higher on the activity series than copper. This means that zinc will be oxidized (lose electrons) and copper ions will be reduced (gain electrons), generating electrical energy.
## Detailed Features Analysis of Galvanic Cells
Galvanic cells, powered by the principles of the chemical activity chart, possess several key features that make them indispensable in modern technology.
### Key Features
1. **Electrode Composition:** The choice of metals for the electrodes directly impacts the cell potential and overall performance. Metals with a significant difference in their activity series ranking are preferred.
* What it is: The electrodes are the physical sites where oxidation and reduction occur. Different metals have different tendencies to lose or gain electrons.
* How it works: The metal higher on the activity series (more reactive) readily loses electrons (oxidation) at the anode, while the metal lower on the activity series (less reactive) readily gains electrons (reduction) at the cathode.
* User Benefit: Selecting appropriate electrode materials ensures a higher cell voltage and a more efficient energy conversion process. This directly translates to longer battery life and improved device performance.
2. **Electrolyte Solution:** The electrolyte provides a medium for ion transport between the electrodes, completing the circuit.
* What it is: The electrolyte is a solution containing ions that can carry electrical charge between the anode and cathode.
* How it works: Ions from the electrolyte migrate to the electrodes to balance the charge generated by the oxidation and reduction reactions. For example, sulfate ions (SO4^2-) might move towards the anode to compensate for the build-up of positive charge as zinc ions (Zn^2+) are released into the solution.
* User Benefit: The electrolyte ensures continuous ion flow, maintaining the electrical circuit and enabling the battery to function properly. Optimizing the electrolyte composition can enhance ion conductivity and improve battery performance.
3. **Salt Bridge:** The salt bridge maintains electrical neutrality in the half-cells by allowing ions to flow between them.
* What it is: A salt bridge is a connection between the two half-cells, often containing an inert electrolyte like potassium chloride (KCl) or sodium nitrate (NaNO3).
* How it works: As oxidation occurs at the anode, positive ions are released into the solution, creating a positive charge imbalance. The salt bridge allows negative ions (e.g., Cl-) to migrate from the salt bridge into the anode half-cell to neutralize this charge. Similarly, positive ions (e.g., K+) migrate from the salt bridge into the cathode half-cell to neutralize the charge imbalance created by the reduction reaction.
* User Benefit: The salt bridge prevents the build-up of charge in the half-cells, ensuring continuous and stable operation of the galvanic cell. Without the salt bridge, the reaction would quickly stop.
4. **Cell Potential (Voltage):** The voltage generated by the galvanic cell is determined by the difference in standard reduction potentials between the two metals.
* What it is: The cell potential is the measure of the electrical potential difference between the anode and cathode, driving the flow of electrons through the external circuit.
* How it works: The greater the difference in reduction potentials, the stronger the driving force for the redox reaction and the higher the cell potential. This potential can be calculated using the Nernst equation under non-standard conditions.
* User Benefit: A higher cell potential means more energy is available to power a device. This translates to longer run times and more efficient energy usage.
5. **Electron Flow:** Electrons flow from the anode (where oxidation occurs) to the cathode (where reduction occurs) through an external circuit.
* What it is: The flow of electrons constitutes the electrical current that powers external devices.
* How it works: Electrons are released at the anode as the metal atoms are oxidized. These electrons travel through the external circuit to the cathode, where they are accepted by the metal ions, causing them to be reduced and deposit onto the cathode.
* User Benefit: The controlled flow of electrons provides a usable source of electrical energy to power various devices and applications.
6. **Spontaneous Reaction:** The redox reaction in a galvanic cell is spontaneous, meaning it occurs without external energy input.
* What it is: Spontaneity refers to the natural tendency of the reaction to proceed without any external driving force.
* How it works: The difference in reduction potentials between the two metals provides the thermodynamic driving force for the reaction. The Gibbs free energy change (ΔG) for the reaction is negative, indicating spontaneity.
* User Benefit: The spontaneous nature of the reaction allows the galvanic cell to generate electrical energy without the need for continuous external power input, making it a convenient and self-contained power source.
7. **Energy Conversion:** Galvanic cells convert chemical energy into electrical energy.
* What it is: The chemical energy stored in the chemical bonds of the reactants (metals) is transformed into electrical energy that can be used to do work.
* How it works: The redox reaction releases energy as electrons are transferred from the anode to the cathode. This energy is harnessed to drive the flow of electrons through the external circuit, generating electrical power.
* User Benefit: Galvanic cells provide a portable and efficient means of converting chemical energy into electrical energy, enabling a wide range of applications from powering small electronic devices to providing backup power for critical systems.
## Significant Advantages, Benefits, and Real-World Value of Galvanic Cells
Galvanic cells, guided by the principles of the chemical activity chart, offer numerous advantages and benefits in various applications.
### User-Centric Value
* **Portability:** Galvanic cells are compact and self-contained, making them ideal for portable electronic devices.
* **Reliability:** They provide a stable and reliable source of power for various applications.
* **Convenience:** They require minimal maintenance and can be easily replaced when depleted.
* **Versatility:** They are available in a wide range of sizes and voltages to suit different needs.
* **Environmental Friendliness (in some cases):** Rechargeable batteries can reduce waste compared to disposable batteries.
### Unique Selling Propositions (USPs)
* **High Energy Density:** Some galvanic cells, like lithium-ion batteries, offer a high energy density, meaning they can store a large amount of energy in a small volume.
* **Long Shelf Life:** Many galvanic cells have a long shelf life, allowing them to be stored for extended periods without significant performance degradation.
* **Quick Recharge Times (for rechargeable batteries):** Modern rechargeable batteries can be quickly recharged, minimizing downtime.
### Evidence of Value
Users consistently report that galvanic cells provide a reliable and convenient power source for their electronic devices. Our analysis reveals that the use of galvanic cells has significantly improved the portability and usability of numerous technologies. For example, the development of lightweight and long-lasting laptops and smartphones would not have been possible without the advancements in galvanic cell technology.
## Comprehensive & Trustworthy Review of Galvanic Cells
Galvanic cells are a cornerstone of modern technology, but like any technology, they have their strengths and weaknesses. This review provides a balanced perspective on their performance, usability, and overall value.
### User Experience & Usability
From our perspective, using devices powered by galvanic cells is generally a seamless experience. Batteries are easy to install and replace (in devices designed for replaceable batteries), and the power delivery is consistent and reliable. Modern devices often incorporate sophisticated battery management systems that optimize energy usage and extend battery life.
### Performance & Effectiveness
Galvanic cells deliver consistent power output until they are depleted. Rechargeable batteries offer the added benefit of being reusable, reducing the need for disposable batteries. However, performance can be affected by factors such as temperature and discharge rate.
### Pros
1. **Portability:** Compact and lightweight, making them ideal for mobile devices.
2. **Reliability:** Provide a consistent and dependable power source.
3. **Versatility:** Available in various sizes, voltages, and chemistries to suit diverse applications.
4. **Rechargeability (for rechargeable types):** Can be recharged and reused multiple times, reducing waste and cost.
5. **High Energy Density (for some types):** Can store a large amount of energy in a small volume, enabling longer run times.
### Cons/Limitations
1. **Limited Lifespan:** Batteries have a finite lifespan and eventually need to be replaced.
2. **Environmental Concerns (for disposable types):** Disposable batteries can contribute to environmental pollution if not properly recycled.
3. **Temperature Sensitivity:** Performance can be affected by extreme temperatures.
4. **Self-Discharge:** Batteries slowly lose charge over time, even when not in use.
### Ideal User Profile
Galvanic cells are best suited for individuals who need a portable and reliable power source for electronic devices, such as smartphones, laptops, and remote controls. They are also ideal for applications where a continuous and stable power supply is critical, such as medical devices and backup power systems.
### Key Alternatives (Briefly)
* **Fuel Cells:** Fuel cells offer higher energy density but are often more complex and expensive.
* **Solar Cells:** Solar cells are environmentally friendly but require sunlight to generate electricity.
### Expert Overall Verdict & Recommendation
Galvanic cells remain a vital power source for a wide range of applications. While they have some limitations, their portability, reliability, and versatility make them an indispensable technology. We recommend using rechargeable batteries whenever possible to minimize environmental impact. For high-power applications, consider exploring advanced battery chemistries like lithium-ion for improved performance.
## Insightful Q&A Section
Here are some insightful questions and expert answers regarding chemical activity charts and their applications:
1. **Q: How does the chemical activity chart help predict corrosion?**
**A:** The activity series indicates which metals are more likely to corrode in a given environment. A metal higher on the chart will corrode preferentially when in contact with a metal lower on the chart in the presence of an electrolyte.
2. **Q: Can the chemical activity chart be used to predict reactions between non-metals?**
**A:** While primarily focused on metals, the activity series can be extended to include some non-metals like hydrogen. However, predicting reactions between non-metals often requires considering other factors like electronegativity and bond strengths.
3. **Q: What factors can affect the accuracy of predictions made using the chemical activity chart?**
**A:** Factors such as non-standard conditions (temperature, concentration), the presence of complexing agents, and the formation of protective oxide layers can influence the actual reactivity of metals and deviate from the predictions based solely on the chart.
4. **Q: How is the chemical activity chart used in the extraction of metals from their ores?**
**A:** A more reactive metal can be used to displace a less reactive metal from its ore. For example, iron (more reactive) is used to extract copper (less reactive) from copper oxide.
5. **Q: Why is hydrogen included in the chemical activity chart?**
**A:** Hydrogen’s position in the activity series is important because it serves as a reference point for determining whether a metal can react with acids to produce hydrogen gas. Metals above hydrogen can displace it from acids.
6. **Q: How does the chemical activity chart relate to the concept of sacrificial anodes in corrosion prevention?**
**A:** Sacrificial anodes are made of a more reactive metal (higher on the activity series) that corrodes preferentially, protecting the less reactive metal structure (e.g., a steel pipeline) to which it is connected.
7. **Q: What are the limitations of using the chemical activity chart in complex chemical systems?**
**A:** The activity series is a simplification and doesn’t account for all factors influencing reaction rates and equilibrium in complex systems. Factors like kinetics, activation energy, and the presence of catalysts can significantly alter reaction outcomes.
8. **Q: How can I use the chemical activity chart to design a corrosion-resistant alloy?**
**A:** By selecting metals that are close together on the activity series and that form stable oxide layers, you can create alloys that are less susceptible to corrosion.
9. **Q: Does the chemical activity chart change over time as new elements are discovered?**
**A:** The fundamental principles remain the same, but the chart can be updated to include new elements and to refine the positions of existing elements based on new experimental data.
10. **Q: What are some common misconceptions about the chemical activity chart?**
**A:** One common misconception is that the activity series guarantees a reaction will occur. It only indicates the likelihood of a reaction under ideal conditions. Other factors, such as kinetics and activation energy, also play a crucial role.
## Conclusion & Strategic Call to Action
The chemical activity chart is a powerful tool for understanding and predicting chemical reactions, particularly those involving metals. Its applications span across various fields, from electrochemistry to corrosion science. By understanding the underlying principles and limitations of the chart, you can effectively use it to solve real-world problems and advance your knowledge of chemistry. Remember that while the chart provides valuable insights, it is essential to consider other factors that can influence reaction outcomes.
As we’ve explored, the chemical activity chart is more than just a list; it’s a key to unlocking the behavior of chemical reactions. Its continued relevance in modern science and technology makes it an indispensable tool for students, researchers, and professionals alike.
Now that you have a comprehensive understanding of the chemical activity chart, we encourage you to explore its applications further. Share your experiences with using the chemical activity chart in the comments below. Explore our advanced guide to electrochemistry for a deeper dive into related concepts. Contact our experts for a consultation on specific chemical applications involving the activity series.
