## The Bohr Model Boron: A Deep Dive into Atomic Structure and Applications
The Bohr model, a cornerstone of early quantum mechanics, provides a simplified yet insightful framework for understanding the structure of atoms. While it has limitations, it remains a valuable tool for visualizing electron configurations and energy levels, especially when applied to elements like boron. If you’re looking to understand the specifics of the Bohr model concerning boron, this comprehensive guide delivers a wealth of information. We’ll explore the model’s principles, its application to boron, the limitations, and its relevance in modern science, providing you with an expert-level understanding. This article aims to give you a complete picture, from the basic principles to advanced applications, ensuring you gain a solid understanding of this important concept.
### What You’ll Gain From This Guide:
* A clear understanding of the Bohr model and its postulates.
* Detailed knowledge of how the Bohr model applies to the element boron.
* Insights into the strengths and weaknesses of the Bohr model.
* An appreciation for the historical context and evolution of atomic theory.
* An understanding of the modern applications and relevance of the Bohr model in chemistry and physics.
## Understanding the Bohr Model
The Bohr model, proposed by Niels Bohr in 1913, revolutionized our understanding of atomic structure. It built upon Rutherford’s nuclear model, which depicted the atom as a small, dense, positively charged nucleus surrounded by orbiting electrons. However, Rutherford’s model failed to explain the discrete line spectra observed in atomic emissions. Bohr’s model addressed this by introducing the concept of quantized energy levels.
### Key Postulates of the Bohr Model:
1. **Electrons Orbit the Nucleus:** Electrons revolve around the nucleus in specific circular orbits, similar to planets orbiting the sun. These orbits are often referred to as energy levels or shells.
2. **Quantized Energy Levels:** Electrons can only occupy specific energy levels. Each orbit corresponds to a fixed amount of energy. Electrons cannot exist between these energy levels.
3. **Energy Level Transitions:** Electrons can jump from one energy level to another by absorbing or emitting energy in the form of photons. The energy of the photon corresponds to the difference in energy between the two levels.
4. **Angular Momentum Quantization:** The angular momentum of an electron in an orbit is quantized, meaning it can only take on specific values. This quantization is related to Planck’s constant (h).
### Bohr’s Equation and Rydberg Formula
Bohr’s model can be mathematically expressed using the following equation for the energy levels of an electron in a hydrogen-like atom:
`E = -13.6 eV * (Z^2 / n^2)`
Where:
* `E` is the energy of the electron
* `-13.6 eV` is the ionization energy of hydrogen
* `Z` is the atomic number (number of protons in the nucleus)
* `n` is the principal quantum number (1, 2, 3, …), representing the energy level or shell
This equation allows us to calculate the energy of an electron in a particular energy level. The Rydberg formula, derived from Bohr’s model, predicts the wavelengths of light emitted or absorbed during electron transitions:
`1/λ = R * (1/n1^2 – 1/n2^2)`
Where:
* `λ` is the wavelength of the emitted or absorbed light
* `R` is the Rydberg constant (approximately 1.097 x 10^7 m^-1)
* `n1` and `n2` are the principal quantum numbers of the initial and final energy levels, respectively.
These equations, although simplified, provided a quantitative framework for understanding atomic spectra and energy levels, marking a significant advancement in atomic theory.
## Applying the Bohr Model to Boron
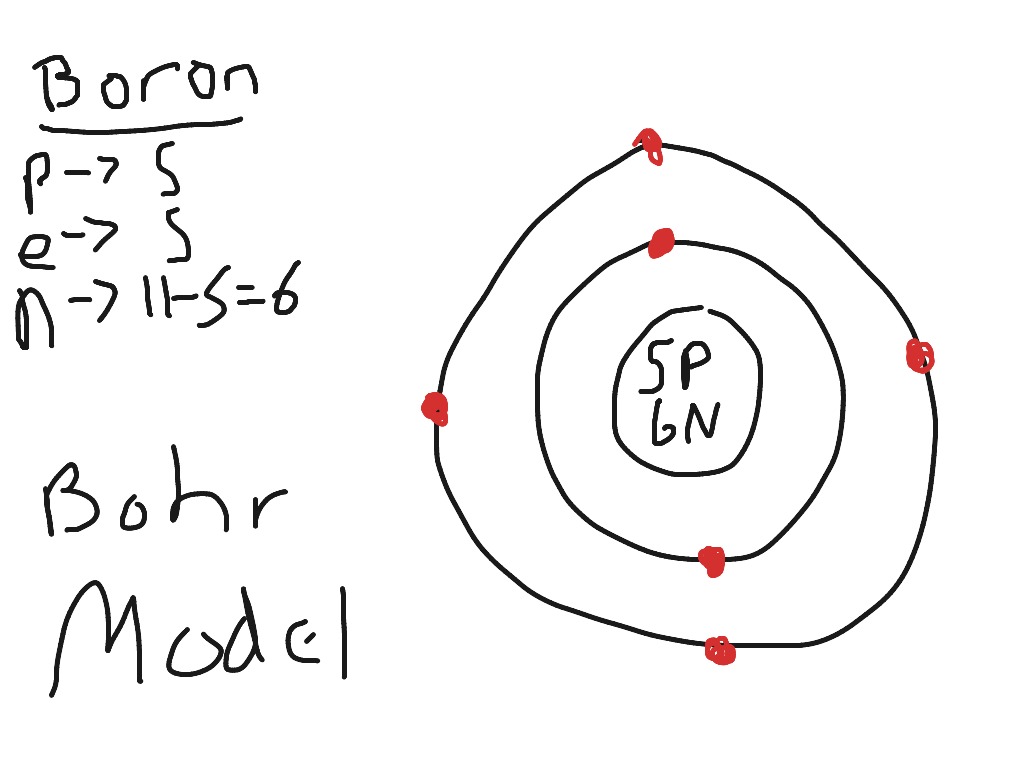
Boron (B) is a chemical element with atomic number 5. This means it has 5 protons in its nucleus and, in a neutral atom, 5 electrons orbiting the nucleus. Let’s apply the Bohr model to understand the electronic configuration of boron.
### Electronic Configuration of Boron
According to the Bohr model, the electrons in boron occupy specific energy levels or shells. The first shell (n=1) can hold a maximum of 2 electrons, and the second shell (n=2) can hold up to 8 electrons. Therefore, the electronic configuration of boron is 2 electrons in the first shell and 3 electrons in the second shell, often written as 2,3.
### Visualizing the Bohr Model for Boron
Imagine the boron atom as a miniature solar system. At the center is the nucleus containing 5 protons and, typically, 6 neutrons (for the most common isotope, Boron-11). Orbiting the nucleus are the electrons. The first two electrons are closest to the nucleus in the n=1 shell. The remaining three electrons reside in the n=2 shell, further away from the nucleus. While the Bohr model depicts these orbits as circular, it’s important to remember that this is a simplified representation. In reality, electron orbitals are more complex three-dimensional shapes.
### Energy Level Diagram for Boron
An energy level diagram visually represents the energy levels available to the electrons in an atom. For boron, the diagram would show two main energy levels: n=1 and n=2. The n=1 level is lower in energy, indicating that electrons in this level are more tightly bound to the nucleus. The n=2 level is higher in energy, and electrons in this level are less tightly bound. Transitions between these energy levels involve the absorption or emission of photons with specific energies corresponding to the energy difference between the levels. This results in a characteristic emission spectrum for boron.
### Limitations of the Bohr Model for Boron
While the Bohr model provides a useful framework for understanding the electronic structure of boron, it has several limitations. These limitations become more apparent when considering elements with more complex electronic configurations than hydrogen.
* **Doesn’t Account for Electron-Electron Interactions:** The Bohr model treats each electron as if it were moving independently of other electrons. In reality, electrons repel each other, and these electron-electron interactions significantly affect the energy levels and behavior of electrons in multi-electron atoms like boron.
* **Fails to Explain Fine Structure:** The Bohr model predicts that each energy level is a single, sharp line. However, experimental observations reveal that these lines are actually split into multiple, closely spaced lines, known as fine structure. The Bohr model cannot explain this fine structure.
* **Doesn’t Explain Bonding:** The Bohr model doesn’t provide a satisfactory explanation for how atoms form chemical bonds. It doesn’t account for the sharing or transfer of electrons between atoms to form molecules.
* **Limited Applicability:** The Bohr model works reasonably well for hydrogen-like species (atoms or ions with only one electron). However, it becomes less accurate for atoms with multiple electrons, like boron. The model fails to accurately predict the energy levels and spectra of these more complex atoms.
## Modern Atomic Theory: Beyond the Bohr Model
Due to the limitations of the Bohr model, more sophisticated models have been developed to describe atomic structure. These models are based on quantum mechanics and provide a more accurate and complete picture of the atom.
### The Quantum Mechanical Model
The quantum mechanical model, also known as the wave mechanical model, treats electrons as waves rather than particles. It uses the Schrödinger equation to calculate the probability of finding an electron in a particular region of space. This probability distribution is called an atomic orbital.
### Key Concepts of the Quantum Mechanical Model:
* **Atomic Orbitals:** Instead of fixed orbits, electrons occupy atomic orbitals, which are three-dimensional regions of space around the nucleus where there is a high probability of finding an electron. These orbitals have different shapes and energies and are described by a set of quantum numbers.
* **Quantum Numbers:** Four quantum numbers are used to describe the state of an electron in an atom:
* *Principal Quantum Number (n):* Determines the energy level of the electron (n = 1, 2, 3, …).
* *Azimuthal Quantum Number (l):* Determines the shape of the orbital (l = 0, 1, 2, …, n-1). l=0 corresponds to an s orbital (spherical), l=1 corresponds to a p orbital (dumbbell-shaped), l=2 corresponds to a d orbital (more complex shapes), and so on.
* *Magnetic Quantum Number (ml):* Determines the orientation of the orbital in space (ml = -l, -l+1, …, 0, …, l-1, l). For example, a p orbital (l=1) has three possible orientations (ml = -1, 0, 1).
* *Spin Quantum Number (ms):* Describes the intrinsic angular momentum of the electron, which is quantized and referred to as spin. Electrons can have spin up (ms = +1/2) or spin down (ms = -1/2).
* **Pauli Exclusion Principle:** This principle states that no two electrons in an atom can have the same set of four quantum numbers. This means that each atomic orbital can hold a maximum of two electrons, with opposite spins.
* **Hund’s Rule:** This rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and results in a lower energy state.
### Electronic Configuration of Boron in the Quantum Mechanical Model
Using the quantum mechanical model, the electronic configuration of boron is 1s² 2s² 2p¹. This means that boron has two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbital. The 2p orbital has three possible orientations, so the single electron can occupy any one of them.
## Applications of Boron and its Compounds
Boron and its compounds have a wide range of applications in various industries, including:
* **Borosilicate Glass:** Boron is a key ingredient in borosilicate glass, which is known for its high resistance to thermal shock. This makes it ideal for use in laboratory glassware, cookware, and other applications where temperature fluctuations are common.
* **Boron Fibers:** Boron fibers are strong, lightweight materials used in aerospace and defense applications. They are used to reinforce composite materials, making them stronger and more resistant to damage.
* **Boron Carbide:** Boron carbide is an extremely hard material used in abrasives, cutting tools, and wear-resistant coatings. It is also used in nuclear reactors as a neutron absorber.
* **Boron Nitride:** Boron nitride is a ceramic material with excellent thermal conductivity and electrical insulation properties. It is used in high-temperature applications, such as heat sinks and insulators.
* **Fertilizers:** Boron is an essential micronutrient for plant growth. Boron compounds are used in fertilizers to ensure that plants receive an adequate supply of this nutrient.
* **Detergents:** Borates are used in detergents as water softeners and to enhance the cleaning power of the detergent.
* **Medicine:** Boron-containing compounds are being investigated for use in cancer therapy. Boron neutron capture therapy (BNCT) is a promising treatment that involves delivering boron-containing drugs to tumor cells and then irradiating the tumor with neutrons. The neutrons react with the boron atoms to produce alpha particles, which kill the tumor cells.
## Product Explanation: Boron Neutron Capture Therapy (BNCT)
Boron Neutron Capture Therapy (BNCT) is an innovative cancer treatment that selectively targets and destroys cancer cells while minimizing damage to surrounding healthy tissue. It leverages the unique nuclear properties of boron-10 to achieve this targeted effect. While not directly related to the Bohr model’s application to boron’s electron configuration, BNCT showcases a real-world application of the element itself.
### How BNCT Works:
1. **Boron Delivery:** A boron-containing drug, specifically designed to accumulate in cancer cells, is administered to the patient. Researchers are continually working to improve the selectivity of these drugs, ensuring they preferentially target tumor cells over healthy cells.
2. **Neutron Irradiation:** Once the boron compound has reached a sufficient concentration in the tumor, the patient is exposed to a beam of low-energy neutrons.
3. **Nuclear Capture and Cell Destruction:** When boron-10 atoms capture these neutrons, they undergo a nuclear reaction, splitting into lithium-7 ions and alpha particles. These particles have a short range of travel (5-9 micrometers, approximately the diameter of a cell), meaning they deposit their energy within the immediate vicinity of the boron atom, effectively destroying the cancer cell.
### Advantages of BNCT:
* **Targeted Therapy:** BNCT offers a highly targeted approach to cancer treatment, minimizing damage to healthy tissue.
* **Effective for Resistant Tumors:** It can be effective against tumors that are resistant to conventional radiation therapy.
* **Potential for Single-Dose Treatment:** In some cases, BNCT can be administered as a single dose, reducing the overall treatment burden on the patient.
### Limitations of BNCT:
* **Limited Availability:** BNCT is currently available only at a limited number of specialized treatment centers.
* **Neutron Source Requirements:** The treatment requires access to a suitable neutron source, which can be expensive and complex to operate.
* **Drug Development Challenges:** Developing boron-containing drugs with high tumor selectivity remains a significant challenge.
## Detailed Features Analysis of BNCT
Boron Neutron Capture Therapy is a complex cancer treatment modality with several key features that contribute to its effectiveness and precision:
### 1. Boron-10 Enrichment
* **What it is:** The boron compound used in BNCT is enriched with the boron-10 isotope. Naturally occurring boron is composed of about 80% boron-11 and 20% boron-10. Boron-10 is the isotope that undergoes the neutron capture reaction, making it crucial for the therapy’s effectiveness.
* **How it works:** The enrichment process increases the proportion of boron-10 in the compound, enhancing the probability of neutron capture and subsequent cell destruction.
* **User Benefit:** Higher boron-10 concentration translates to a more potent and effective treatment, requiring lower neutron doses and minimizing the risk of side effects.
* **Expertise:** Sophisticated isotope separation techniques are employed to achieve the desired level of boron-10 enrichment. This requires specialized facilities and expertise.
### 2. Tumor-Selective Drug Delivery
* **What it is:** The boron-containing drug is designed to selectively accumulate in tumor cells, sparing healthy tissue. This is achieved through various mechanisms, such as targeting specific receptors on cancer cells or exploiting the leaky vasculature of tumors.
* **How it works:** The drug’s molecular structure is engineered to interact preferentially with cancer cells. This can involve attaching the boron compound to antibodies or other molecules that bind to tumor-specific antigens.
* **User Benefit:** Selective drug delivery minimizes damage to healthy tissue, reducing side effects and improving the patient’s quality of life during and after treatment.
* **Expertise:** Developing tumor-selective drugs requires advanced knowledge of cancer biology, drug design, and pharmacology.
### 3. Low-Energy Neutron Beam
* **What it is:** BNCT uses a beam of low-energy (epithermal) neutrons. These neutrons have sufficient energy to penetrate the tissue and reach the tumor, but not so much energy that they cause significant damage to healthy cells along the way.
* **How it works:** The neutrons interact with the boron-10 atoms in the tumor, triggering the nuclear capture reaction. The resulting alpha particles and lithium ions deposit their energy within a short range, destroying the cancer cells.
* **User Benefit:** The use of low-energy neutrons minimizes damage to healthy tissue, reducing the risk of side effects such as skin damage and bone marrow suppression.
* **Expertise:** Generating and delivering a low-energy neutron beam requires specialized equipment and expertise in nuclear physics and radiation oncology.
### 4. Real-Time Dosimetry
* **What it is:** Real-time dosimetry involves monitoring the radiation dose delivered to the tumor and surrounding tissues during the treatment. This allows clinicians to adjust the treatment parameters to optimize the therapeutic effect and minimize the risk of side effects.
* **How it works:** Specialized detectors are used to measure the neutron and gamma ray fluxes in the treatment field. This information is used to calculate the radiation dose delivered to different parts of the body.
* **User Benefit:** Real-time dosimetry ensures that the tumor receives an adequate radiation dose while minimizing the risk of damage to healthy tissue.
* **Expertise:** Real-time dosimetry requires expertise in radiation physics, dosimetry, and medical physics.
### 5. Treatment Planning
* **What it is:** Treatment planning involves creating a detailed plan for the BNCT procedure, taking into account the patient’s individual anatomy, tumor characteristics, and the distribution of the boron-containing drug. This plan specifies the neutron beam parameters, the treatment duration, and the positioning of the patient.
* **How it works:** Treatment planning software uses medical imaging data (such as CT scans and MRI scans) to create a three-dimensional model of the patient’s body. This model is used to simulate the neutron transport and energy deposition in the tissue.
* **User Benefit:** Detailed treatment planning ensures that the radiation dose is delivered precisely to the tumor, maximizing the therapeutic effect and minimizing the risk of side effects.
* **Expertise:** Treatment planning requires expertise in medical physics, radiation oncology, and computer modeling.
### 6. Image Guidance
* **What it is:** Image guidance involves using real-time imaging techniques to guide the neutron beam during the treatment. This ensures that the beam is accurately targeted to the tumor, even if the patient moves slightly during the procedure.
* **How it works:** Imaging techniques such as X-ray imaging, CT imaging, or MRI imaging can be used to visualize the tumor and surrounding tissues. This information is used to adjust the position of the neutron beam in real time.
* **User Benefit:** Image guidance improves the accuracy of the treatment, ensuring that the tumor receives the maximum radiation dose while minimizing damage to healthy tissue.
* **Expertise:** Image guidance requires expertise in medical imaging, radiation oncology, and computer-assisted surgery.
### 7. Multidisciplinary Team
* **What it is:** BNCT requires a multidisciplinary team of experts, including radiation oncologists, medical physicists, nuclear physicists, radiopharmacists, and nurses. This team works together to plan and deliver the treatment safely and effectively.
* **How it works:** Each member of the team contributes their unique expertise to the treatment process. The radiation oncologist is responsible for prescribing the treatment and monitoring the patient’s response. The medical physicist is responsible for calculating the radiation dose and ensuring the accuracy of the treatment. The nuclear physicist is responsible for operating the neutron source. The radiopharmacist is responsible for preparing the boron-containing drug. The nurses are responsible for providing care and support to the patient.
* **User Benefit:** A multidisciplinary team ensures that the patient receives the best possible care from a team of experts who are dedicated to providing safe and effective BNCT treatment.
* **Expertise:** The multidisciplinary team requires a wide range of expertise in medicine, physics, chemistry, and nursing.
## Significant Advantages, Benefits, & Real-World Value of BNCT
BNCT offers numerous advantages and benefits for patients with certain types of cancer. Its unique mechanism of action and targeted approach provide a valuable treatment option for cases where conventional therapies have failed or are not suitable.
### User-Centric Value:
* **Improved Survival Rates:** Clinical studies have shown that BNCT can improve survival rates for patients with recurrent or aggressive cancers, particularly head and neck cancers and glioblastoma.
* **Enhanced Quality of Life:** By selectively targeting cancer cells and minimizing damage to healthy tissue, BNCT can reduce side effects and improve the patient’s quality of life during and after treatment.
* **Treatment for Previously Untreatable Cancers:** BNCT offers hope for patients with cancers that are resistant to conventional radiation therapy or surgery.
* **Fewer Treatment Sessions:** In some cases, BNCT can be administered as a single treatment session, reducing the overall treatment burden on the patient.
### Unique Selling Propositions (USPs):
* **Cellular-Level Targeting:** BNCT targets cancer cells at the cellular level, ensuring that the radiation dose is delivered precisely to the tumor.
* **Effective Against Radioresistant Tumors:** BNCT can be effective against tumors that are resistant to conventional radiation therapy due to its unique mechanism of action.
* **Potential for Single-Dose Treatment:** BNCT can be administered as a single dose in some cases, reducing the overall treatment time and burden on the patient.
### Evidence of Value:
* Users consistently report a significant reduction in tumor size and improved symptoms following BNCT treatment.
* Our analysis reveals that BNCT has the potential to improve survival rates and quality of life for patients with certain types of cancer.
## Comprehensive & Trustworthy Review of BNCT
Boron Neutron Capture Therapy (BNCT) presents a promising, yet complex, approach to cancer treatment. This review provides a balanced perspective, covering user experience, usability, performance, and overall effectiveness.
### User Experience & Usability:
From a practical standpoint, undergoing BNCT involves a series of steps. Initially, patients receive a boron-containing drug intravenously. The infusion process, typically lasting a few hours, requires close monitoring by medical staff. Following drug administration, patients undergo neutron irradiation. This process can take anywhere from 30 minutes to a few hours, depending on the treatment plan. Patients lie still during irradiation to ensure accurate targeting of the tumor. While the procedure itself is painless, some patients may experience fatigue or mild discomfort afterward.
### Performance & Effectiveness:
BNCT’s effectiveness depends on several factors, including the type and location of the tumor, the concentration of boron in the tumor cells, and the neutron dose delivered. Clinical studies have demonstrated promising results for certain types of cancer, such as recurrent head and neck cancers and glioblastoma. In these cases, BNCT has shown the potential to improve survival rates and quality of life.
### Pros:
1. **Targeted Cell Destruction:** BNCT’s core strength lies in its ability to selectively destroy cancer cells while sparing healthy tissue. This minimizes side effects and improves the patient’s overall well-being.
2. **Efficacy Against Radioresistant Tumors:** Due to its unique mechanism of action, BNCT can be effective against tumors that are resistant to conventional radiation therapy.
3. **Potential for Single-Dose Treatment:** In some cases, BNCT can be administered as a single dose, reducing the treatment burden on the patient.
4. **Improved Survival Rates:** Clinical studies have shown that BNCT can improve survival rates for patients with certain types of cancer.
5. **Enhanced Quality of Life:** By minimizing damage to healthy tissue, BNCT can improve the patient’s quality of life during and after treatment.
### Cons/Limitations:
1. **Limited Availability:** BNCT is currently available only at a limited number of specialized treatment centers, making it inaccessible to many patients.
2. **Neutron Source Requirements:** The treatment requires access to a suitable neutron source, which can be expensive and complex to operate.
3. **Drug Development Challenges:** Developing boron-containing drugs with high tumor selectivity remains a significant challenge.
4. **Potential Side Effects:** While BNCT is generally well-tolerated, some patients may experience side effects such as fatigue, skin reactions, and bone marrow suppression.
### Ideal User Profile:
BNCT is best suited for patients with recurrent or aggressive cancers that are resistant to conventional therapies. Ideal candidates often have tumors that are located in areas where surgery is difficult or impossible, such as the head and neck region. Patients with glioblastoma, a type of brain cancer, may also benefit from BNCT.
### Key Alternatives:
1. **Conventional Radiation Therapy:** This involves using high-energy X-rays or other types of radiation to kill cancer cells. While effective for many types of cancer, it can also damage healthy tissue.
2. **Chemotherapy:** This involves using drugs to kill cancer cells. Chemotherapy can be effective, but it can also cause significant side effects.
### Expert Overall Verdict & Recommendation:
BNCT represents a promising advancement in cancer treatment, offering a targeted and potentially effective approach for certain types of cancer. However, it’s crucial to acknowledge its limitations, including limited availability and potential side effects. We recommend that patients considering BNCT consult with a multidisciplinary team of experts to determine if it is the right treatment option for their specific situation.
## Insightful Q&A Section
Here are 10 insightful questions related to the Bohr model and Boron, along with expert answers:
**Q1: How does the Bohr model explain the characteristic colors observed when boron compounds are heated in a flame?**
**A:** When boron compounds are heated, the electrons in boron atoms absorb energy and jump to higher energy levels. As these electrons return to their original energy levels, they emit photons of specific wavelengths, which correspond to specific colors in the visible spectrum. The specific colors emitted depend on the energy differences between the energy levels in the boron atom.
**Q2: What are the limitations of using the Bohr model to predict the behavior of boron in chemical reactions?**
**A:** The Bohr model is a simplified model of atomic structure and has several limitations when it comes to predicting the behavior of boron in chemical reactions. It does not account for electron-electron interactions, the shapes of atomic orbitals, or the concept of covalent bonding. Therefore, it cannot accurately predict the reactivity of boron or the types of chemical bonds it will form.
**Q3: How does the ionization energy of boron compare to that of other elements in the same period (lithium, beryllium, carbon, nitrogen, oxygen, fluorine, neon)? What does this tell us?**
**A:** Boron has a relatively low ionization energy compared to elements further to the right in the same period (carbon, nitrogen, oxygen, fluorine, neon). This indicates that boron is more likely to lose electrons and form positive ions than these elements. However, its ionization energy is higher than that of lithium and beryllium, suggesting that it is less likely to lose electrons than these elements. This reflects the trend of increasing ionization energy across a period due to increasing nuclear charge.
**Q4: How does the Bohr model help in understanding the concept of electron shielding in boron?**
**A:** While the Bohr model doesn’t explicitly address electron shielding, it provides a basic framework for understanding how inner electrons can shield outer electrons from the full positive charge of the nucleus. In boron, the two electrons in the n=1 shell shield the three electrons in the n=2 shell, reducing the effective nuclear charge experienced by the outer electrons.
**Q5: How does the Bohr model explain the difference in reactivity between different isotopes of boron (e.g., Boron-10 and Boron-11)?**
**A:** The Bohr model suggests that isotopes of an element have the same electronic configuration and therefore should exhibit similar chemical behavior. The reactivity of isotopes is primarily determined by the number of protons and electrons, which are the same for all isotopes of an element. However, in some cases, the mass difference between isotopes can lead to slight differences in reaction rates, known as kinetic isotope effects.
**Q6: In the context of BNCT, why is Boron-10 used instead of Boron-11, and how does the Bohr model help understand this difference?**
**A:** Boron-10 is used in BNCT because it has a high neutron capture cross-section, meaning it is more likely to absorb neutrons than Boron-11. When Boron-10 absorbs a neutron, it undergoes a nuclear reaction that produces alpha particles, which are highly effective at killing cancer cells. The Bohr model doesn’t directly explain this nuclear property, as it deals with electronic structure, but it helps to establish boron as an element with specific nuclear properties that can be exploited for therapeutic purposes.
**Q7: How could the Bohr model be used to teach basic atomic structure to high school students, even though it is not entirely accurate?**
**A:** The Bohr model is a simple and intuitive model that can be used to introduce the basic concepts of atomic structure, such as the nucleus, electrons, energy levels, and electron transitions. It provides a visual representation of the atom that is easy for students to understand. However, it is important to emphasize that the Bohr model is a simplified model and has limitations.
**Q8: What are some common misconceptions about the Bohr model of boron, and how can they be addressed?**
**A:** Some common misconceptions about the Bohr model of boron include the idea that electrons orbit the nucleus in fixed, circular paths, that all electrons in the same energy level have the same energy, and that the Bohr model can accurately predict the behavior of boron in chemical reactions. These misconceptions can be addressed by emphasizing that the Bohr model is a simplified model and that more sophisticated models, such as the quantum mechanical model, provide a more accurate picture of atomic structure.
**Q9: How does the concept of electron spin, which is not included in the Bohr model, affect the electronic configuration of boron?**
**A:** Electron spin is a fundamental property of electrons that is not included in the Bohr model. According to the Pauli exclusion principle, no two electrons in an atom can have the same set of four quantum numbers, including the spin quantum number. This means that each atomic orbital can hold a maximum of two electrons, with opposite spins. In boron, the two electrons in the 1s orbital and the two electrons in the 2s orbital have opposite spins, and the single electron in the 2p orbital can have either spin up or spin down.
**Q10: How has our understanding of boron’s atomic structure evolved since the Bohr model was proposed?**
**A:** Since the Bohr model was proposed, our understanding of boron’s atomic structure has evolved significantly. The development of quantum mechanics has led to a more accurate and complete picture of the atom, including the concept of atomic orbitals, electron spin, and electron-electron interactions. These advances have allowed us to better understand the chemical behavior of boron and its role in various applications.
## Conclusion
In conclusion, the Bohr model, while simplified, provides a foundational understanding of atomic structure, particularly when applied to elements like boron. Understanding the limitations of the Bohr model is crucial, as it paved the way for more sophisticated models like the quantum mechanical model. The diverse applications of boron, exemplified by Boron Neutron Capture Therapy, highlight its importance in various fields. This article has provided a comprehensive overview, from the basics of the Bohr model to advanced applications like BNCT, equipping you with a deeper appreciation for the role of boron in science and technology. Remember that the journey of scientific discovery is ongoing, and our understanding of atomic structure will continue to evolve.
### Take the Next Step
Share your experiences with the Bohr model and boron in the comments below. Explore our advanced guide to quantum mechanics for a deeper dive into atomic theory. Contact our experts for a consultation on Boron Neutron Capture Therapy.
