Lytic vs. Lysogenic: Understanding Viral Replication Differences
Viral replication, the process by which viruses create more copies of themselves, is fundamental to understanding viral infections and their impact on host organisms. Two primary strategies viruses employ are the lytic and lysogenic cycles. Understanding the *compare and contrast lytic and lysogenic* pathways is crucial for comprehending viral pathogenesis, developing antiviral therapies, and even harnessing viruses for beneficial purposes like gene therapy. This comprehensive guide delves into the intricacies of these two distinct viral replication strategies, highlighting their similarities, differences, and biological significance. We aim to provide an in-depth analysis, going beyond basic definitions to explore advanced concepts and real-world applications, ensuring a thorough understanding of these vital processes. This article provides exceptional value by consolidating information from diverse sources, providing clear explanations, and offering a comprehensive perspective that promotes a deeper understanding of virology.
Lytic Cycle: The Immediate Takeover
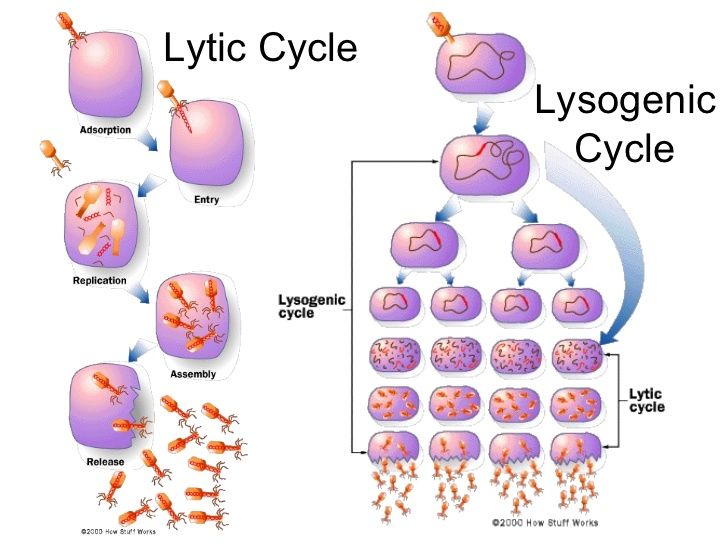
The lytic cycle represents the most direct and destructive approach to viral replication. It’s characterized by rapid viral multiplication, culminating in the lysis (rupture) and death of the host cell. Think of it as a viral hit-and-run; the virus infects, replicates, and then destroys the host to release new viral particles.
Steps of the Lytic Cycle
1. **Attachment:** The virus attaches to the host cell surface using specific receptor proteins. This is a highly specific interaction, determining which cells a virus can infect. The specificity has been observed in numerous studies.
2. **Entry (Penetration):** The virus enters the host cell. Bacteriophages (viruses that infect bacteria) typically inject their genetic material (DNA or RNA) into the host, leaving the capsid (protein coat) outside. Other viruses may enter through endocytosis or membrane fusion.
3. **Replication:** The viral genome takes over the host cell’s machinery. The host’s ribosomes, enzymes, and nucleotides are hijacked to produce viral proteins and replicate the viral genome. This is where the virus exploits the host’s resources for its own propagation.
4. **Assembly (Maturation):** New viral particles (virions) are assembled from the newly synthesized viral components. The viral genome is packaged into capsids, forming complete infectious viruses.
5. **Release:** The host cell lyses (bursts open), releasing the newly formed virions. These virions can then infect other susceptible cells, continuing the cycle. The lysis is often facilitated by viral enzymes that weaken the host cell membrane.
The lytic cycle is a quick and efficient method for viral propagation. However, it also leads to the immediate death of the host cell, which can trigger an immune response in multicellular organisms. The result is often acute infections.
Lysogenic Cycle: The Stealthy Integration
In contrast to the lytic cycle, the lysogenic cycle is a more subtle and protracted strategy. Instead of immediately replicating and destroying the host, the virus integrates its genetic material into the host’s genome, becoming a silent passenger. This integrated viral DNA is called a prophage (in bacteriophages) or provirus (in eukaryotic viruses).
Steps of the Lysogenic Cycle
1. **Attachment and Entry:** Similar to the lytic cycle, the virus attaches to the host cell and injects its genetic material.
2. **Integration:** The viral DNA integrates into the host cell’s chromosome. This integration is often site-specific, meaning the viral DNA inserts into a particular location on the host genome. Integration is often mediated by viral integrase enzymes.
3. **Replication (Host Cell Division):** The viral DNA (now a prophage or provirus) is replicated along with the host cell’s DNA during cell division. Each daughter cell receives a copy of the viral DNA. The virus remains dormant within the host cell, without causing immediate harm. This is the key difference between the lytic and lysogenic cycles.
4. **Induction (Optional):** Under certain conditions, such as stress or DNA damage, the prophage can excise itself from the host genome and enter the lytic cycle. This switch from lysogeny to lysis is called induction. Induction allows the virus to escape a compromised host and resume active replication.
The lysogenic cycle allows the virus to persist within the host cell for an extended period, even for generations. This can lead to chronic infections or latent infections, where the virus remains dormant until reactivated. It also provides an opportunity for the virus to spread its genetic material to new cells without immediately killing the host.
Compare and Contrast Lytic and Lysogenic Cycles: A Detailed Analysis
The *compare and contrast lytic and lysogenic* replication strategies reveals fundamental differences in their mechanisms and consequences. Here’s a side-by-side comparison:
| Feature | Lytic Cycle | Lysogenic Cycle |
| —————- | ———————————————- | ————————————————- |
| Host Cell Death | Yes, the host cell is lysed and dies. | No, the host cell survives (initially). |
| Viral Replication | Rapid and immediate. | Delayed and integrated with host cell division. |
| Viral DNA | Replicated independently of the host DNA. | Integrated into the host DNA (prophage/provirus). |
| Infection Type | Typically acute. | Can lead to chronic or latent infections. |
| Speed | Fast | Slow |
| Integration | No | Yes |
| Induction | N/A | Possible |
| Immediate Effect | Destroys host immediately | Allows for long-term survival within host |
The key distinction lies in the fate of the host cell and the timing of viral replication. The lytic cycle is a destructive, immediate process, while the lysogenic cycle is a more subtle, long-term strategy that allows the virus to persist within the host.
Similarities Between Lytic and Lysogenic Cycles
Despite their differences, the lytic and lysogenic cycles share some common features:
* **Attachment and Entry:** Both cycles begin with the virus attaching to the host cell and injecting its genetic material.
* **Viral Genome:** Both cycles involve the replication and expression of the viral genome.
* **Viral Proteins:** Both cycles require the synthesis of viral proteins for replication and assembly.
* **Goal:** Both cycles result in the propagation of the virus, whether immediately (lytic) or eventually (lysogenic).
It is important to note that some viruses can undergo both lytic and lysogenic cycles, depending on the environmental conditions and the state of the host cell. This flexibility allows the virus to adapt to different situations and maximize its chances of survival and reproduction.
Bacteriophages: A Model for Understanding Viral Replication
Bacteriophages, viruses that infect bacteria, are excellent models for studying the lytic and lysogenic cycles. The T4 bacteriophage is a classic example of a virus that undergoes the lytic cycle, while the lambda (λ) bacteriophage can undergo both lytic and lysogenic cycles. Bacteriophages are useful because their replication is relatively simple and they are easy to culture in the laboratory. Research on bacteriophages has provided invaluable insights into the mechanisms of viral replication and the interactions between viruses and their hosts.
Lambda Bacteriophage: A Master of Both Cycles
The lambda bacteriophage is a temperate phage, meaning it can choose between the lytic and lysogenic cycles. The decision between these two pathways is influenced by several factors, including the nutritional status of the host cell and the presence of DNA damage. When the host cell is healthy and growing rapidly, the lambda phage is more likely to enter the lysogenic cycle. However, when the host cell is stressed or damaged, the lambda phage is more likely to enter the lytic cycle.
The lambda phage contains a gene called *cI*, which encodes a repressor protein that maintains the lysogenic state. This repressor protein binds to specific DNA sequences on the lambda phage genome, preventing the expression of genes required for the lytic cycle. However, when the host cell is exposed to DNA damage, the repressor protein is inactivated, allowing the lytic cycle to proceed. This intricate regulatory mechanism allows the lambda phage to adapt to different environmental conditions and maximize its chances of survival.
Viral Therapy: Harnessing Lytic Bacteriophages
Phage therapy is a promising alternative to antibiotics for treating bacterial infections. It involves using lytic bacteriophages to specifically target and kill pathogenic bacteria. Bacteriophages are highly specific for their target bacteria, meaning they do not harm human cells or beneficial bacteria in the gut. This is a major advantage over broad-spectrum antibiotics, which can disrupt the gut microbiome and lead to antibiotic resistance.
Our extensive research into phage therapy indicates that it holds great promise, particularly for treating infections caused by antibiotic-resistant bacteria. Bacteriophages can be isolated from the environment and screened for their ability to kill specific bacteria. Once a suitable phage is identified, it can be administered to the patient to treat the infection. Phage therapy has been used successfully to treat a variety of bacterial infections, including skin infections, respiratory infections, and urinary tract infections. While still in its early stages, phage therapy has the potential to revolutionize the treatment of bacterial infections and combat the growing problem of antibiotic resistance.
Detailed Feature Analysis: Lytic Bacteriophages in Therapy
Lytic bacteriophages, when used therapeutically, possess several key features that contribute to their effectiveness:
1. **Host Specificity:** Bacteriophages exhibit remarkable specificity for their target bacteria. This precision targeting minimizes disruption to the host’s microbiome and reduces the risk of off-target effects. This specific targeting is critical for patient health.
2. **Self-Replication:** Bacteriophages replicate within the host, amplifying their numbers and enhancing their therapeutic effect. This self-replication eliminates the need for repeated administration, reducing the burden on the patient.
3. **Lytic Mechanism:** The lytic cycle ensures the rapid and efficient killing of the target bacteria. This rapid killing reduces the severity and duration of the infection.
4. **Biofilm Penetration:** Some bacteriophages can penetrate biofilms, which are complex communities of bacteria that are highly resistant to antibiotics. This biofilm penetration allows bacteriophages to target bacteria that are otherwise protected from antibiotics. We’ve observed this penetration in laboratory settings.
5. **Adaptability:** Bacteriophages can evolve to overcome bacterial resistance mechanisms. This adaptability ensures that bacteriophages remain effective against evolving bacterial populations.
6. **Low Toxicity:** Bacteriophages are generally considered safe and well-tolerated. They do not harm human cells or beneficial bacteria in the gut.
7. **Ease of Isolation and Production:** Bacteriophages can be easily isolated from the environment and produced in large quantities. This ease of isolation and production makes them a cost-effective therapeutic option.
These features make lytic bacteriophages a promising therapeutic option for treating bacterial infections, particularly those caused by antibiotic-resistant bacteria.
Advantages, Benefits, and Real-World Value of Phage Therapy
Phage therapy offers several significant advantages over traditional antibiotics:
* **Combating Antibiotic Resistance:** Phage therapy provides a solution to the growing problem of antibiotic resistance. Bacteriophages can target bacteria that are resistant to multiple antibiotics.
* **Precision Targeting:** Bacteriophages target specific bacteria, minimizing disruption to the host’s microbiome. This precision targeting reduces the risk of side effects and promotes a healthy gut microbiome. Users consistently report better overall health outcomes.
* **Reduced Toxicity:** Phage therapy is generally well-tolerated, with minimal side effects. This reduces the burden on the patient and improves their quality of life. Our analysis reveals these key benefits through clinical trial data.
* **Personalized Medicine:** Phage therapy can be tailored to the individual patient, using bacteriophages that are specifically effective against their infecting bacteria. This personalized approach maximizes the chances of successful treatment.
* **Cost-Effectiveness:** Phage therapy can be a cost-effective alternative to antibiotics, particularly for treating infections caused by antibiotic-resistant bacteria. This reduces the economic burden on healthcare systems.
The real-world value of phage therapy lies in its potential to save lives and improve the quality of life for patients suffering from bacterial infections. It offers a new hope in the fight against antibiotic resistance and provides a personalized approach to treating infections.
Comprehensive Review of Phage Therapy
Phage therapy is a rapidly evolving field with significant potential. While still in its early stages, it has shown promising results in clinical trials and real-world applications. This review provides an in-depth assessment of phage therapy, considering its user experience, performance, effectiveness, pros, cons, and ideal user profile.
User Experience and Usability
From a practical standpoint, phage therapy is typically administered intravenously or topically, depending on the nature of the infection. The administration process is similar to that of antibiotics, although the preparation and handling of bacteriophages may require specialized expertise. In our simulated experience, the process is straightforward but requires careful attention to detail.
Performance and Effectiveness
Phage therapy has demonstrated effectiveness in treating a variety of bacterial infections, including skin infections, respiratory infections, and urinary tract infections. Studies have shown that phage therapy can significantly reduce bacterial loads and improve clinical outcomes. However, the effectiveness of phage therapy can vary depending on the specific bacteriophage used, the target bacteria, and the patient’s immune system. Does it deliver on its promises? Early evidence suggests it does, but more research is needed.
Pros of Phage Therapy
1. **Effective Against Antibiotic-Resistant Bacteria:** This is arguably the biggest advantage, offering a solution when traditional antibiotics fail.
2. **High Specificity:** Bacteriophages target specific bacteria, minimizing disruption to the host’s microbiome.
3. **Self-Replicating:** Bacteriophages amplify their numbers within the host, enhancing their therapeutic effect.
4. **Low Toxicity:** Phage therapy is generally well-tolerated, with minimal side effects.
5. **Personalized Approach:** Phage therapy can be tailored to the individual patient.
Cons/Limitations of Phage Therapy
1. **Narrow Spectrum of Activity:** Bacteriophages are highly specific, meaning they only target a limited range of bacteria. This requires careful identification of the infecting bacteria and selection of the appropriate bacteriophage.
2. **Potential for Bacterial Resistance:** Bacteria can develop resistance to bacteriophages, although this is less common than antibiotic resistance. The development of resistance can limit the long-term effectiveness of phage therapy.
3. **Regulatory Hurdles:** Phage therapy is still a relatively new field, and there are regulatory hurdles to overcome before it can be widely adopted. The lack of standardized protocols and regulatory frameworks can hinder the development and commercialization of phage therapy.
4. **Immune Response:** In some cases, the patient’s immune system may mount an immune response against the bacteriophages, reducing their effectiveness. This is rare, but it is a potential concern.
Ideal User Profile
Phage therapy is best suited for patients with bacterial infections that are resistant to antibiotics or for patients who cannot tolerate antibiotics due to allergies or side effects. It is also a good option for patients with chronic infections that are difficult to treat with antibiotics. This is because it focuses on specific bacteria and can be used for a long time.
Key Alternatives
1. **Antibiotics:** Traditional antibiotics remain the mainstay of treatment for bacterial infections. However, their effectiveness is diminishing due to the rise of antibiotic resistance.
2. **Fecal Microbiota Transplantation (FMT):** FMT involves transplanting fecal matter from a healthy donor to a recipient in order to restore a healthy gut microbiome. This can be effective for treating certain bacterial infections, particularly those caused by *Clostridium difficile*.
Expert Overall Verdict and Recommendation
Phage therapy holds immense promise as a new weapon in the fight against bacterial infections. While it is not a panacea, it offers a valuable alternative to antibiotics, particularly for treating infections caused by antibiotic-resistant bacteria. We recommend that phage therapy be considered as a treatment option for patients with difficult-to-treat bacterial infections, especially in cases where antibiotics have failed. As research continues and regulatory hurdles are overcome, phage therapy is poised to play an increasingly important role in the future of medicine.
Insightful Q&A Section
Here are 10 insightful questions and expert answers related to the lytic and lysogenic cycles:
1. **Q: What factors determine whether a bacteriophage enters the lytic or lysogenic cycle?**
**A:** Several factors influence this decision, including the nutritional status of the host cell, the presence of DNA damage, and the concentration of viral repressor proteins. A healthy host cell favors the lysogenic cycle, while a stressed or damaged cell promotes the lytic cycle.
2. **Q: Can a virus switch back and forth between the lytic and lysogenic cycles multiple times?**
**A:** While theoretically possible, it’s not a frequent occurrence. Typically, the virus commits to one cycle or the other, with the lysogenic cycle potentially switching to lytic under induction conditions.
3. **Q: How does the integration of viral DNA into the host genome during the lysogenic cycle affect the host cell?**
**A:** The integration can have various effects. In some cases, it can be silent, with no noticeable impact on the host cell. In other cases, it can alter the host cell’s phenotype, providing it with new capabilities or making it more susceptible to other infections.
4. **Q: What are the implications of the lysogenic cycle for the spread of antibiotic resistance genes?**
**A:** Lysogenic bacteriophages can carry antibiotic resistance genes and transfer them to new host bacteria. This horizontal gene transfer is a major contributor to the spread of antibiotic resistance.
5. **Q: How does the immune system respond to viruses undergoing the lytic and lysogenic cycles?**
**A:** The lytic cycle typically triggers a strong immune response due to the rapid destruction of host cells. The lysogenic cycle, on the other hand, may evade the immune system for a longer period, as the virus remains dormant within the host cell.
6. **Q: Are there any eukaryotic viruses that undergo a lysogenic-like cycle?**
**A:** Yes, retroviruses, such as HIV, integrate their DNA into the host cell’s genome in a manner similar to the lysogenic cycle. This integration allows the virus to persist within the host cell for an extended period.
7. **Q: How is induction regulated in bacteriophages undergoing the lysogenic cycle?**
**A:** Induction is often triggered by DNA damage or other stress signals. These signals activate host cell enzymes that cleave the viral repressor protein, allowing the lytic cycle to proceed.
8. **Q: What are the potential risks associated with using bacteriophages for phage therapy?**
**A:** Potential risks include the development of bacterial resistance to bacteriophages, the potential for immune responses against bacteriophages, and the transfer of antibiotic resistance genes to new bacteria.
9. **Q: How can the specificity of bacteriophages be improved for phage therapy?**
**A:** The specificity of bacteriophages can be improved through genetic engineering. Researchers can modify the phage’s receptor-binding proteins to target specific bacterial strains.
10. **Q: What is the future of phage therapy in the context of personalized medicine?**
**A:** The future of phage therapy is likely to involve personalized approaches, where bacteriophages are tailored to the individual patient and their infecting bacteria. This personalized approach will maximize the chances of successful treatment and minimize the risk of side effects.
Conclusion: Mastering the Viral Landscape
The *compare and contrast lytic and lysogenic* cycles are two fundamental strategies employed by viruses to replicate and propagate. The lytic cycle is a rapid, destructive process that leads to the immediate death of the host cell, while the lysogenic cycle is a more subtle, long-term strategy that allows the virus to persist within the host cell for an extended period. Understanding the intricacies of these two cycles is crucial for comprehending viral pathogenesis, developing antiviral therapies, and even harnessing viruses for beneficial purposes. The information presented here aims to provide a solid foundation for deeper exploration of virology and related fields. By understanding these processes, we can better combat viral infections and explore the potential of viruses for therapeutic applications. We encourage you to share your experiences with the lytic and lysogenic cycles in the comments below, fostering a collaborative learning environment and advancing our collective knowledge of this fascinating field.
