What’s Freezing Point in Fahrenheit? A Comprehensive Guide
Navigating the world often involves understanding basic scientific concepts, and one of the most fundamental is the freezing point of water. When we talk about “what’s freezing point in fahrenheit,” we’re asking a question that has implications for everything from weather forecasting to cooking. This article aims to provide an exhaustive exploration of this topic, ensuring you not only know the answer but also understand the science behind it, its real-world applications, and why it matters. We’ll delve into the nuances, related concepts, and even explore some common misconceptions. Our goal is to offer a resource that is not only informative but also trustworthy and authoritative, reflecting our deep expertise in the subject.
Understanding the Basics: What is Freezing Point in Fahrenheit?
The freezing point, in general, is the temperature at which a liquid transitions into a solid state. More specifically, *what’s freezing point in Fahrenheit* refers to the temperature at which water freezes when measured on the Fahrenheit scale. This is a fixed point used in various scientific and practical applications.
The Precise Definition
Water freezes at 32 degrees Fahrenheit (32°F). This means that when the temperature of water drops to or below this point, under normal atmospheric pressure, it will begin to turn into ice. It’s crucial to note that this is for pure water. The presence of impurities, such as salt, can lower the freezing point – a concept we’ll explore later.
A Historical Perspective
The Fahrenheit scale was developed by Daniel Gabriel Fahrenheit in the early 18th century. He based his scale on two fixed points: the freezing point of a salt-water mixture (set at 0°F) and the human body temperature (originally set at 90°F, later revised). While the Fahrenheit scale is still widely used in the United States, it’s important to understand its historical context to appreciate the significance of the freezing point in this system.
Why is the Freezing Point Important?
Understanding the freezing point of water is essential for several reasons:
* **Weather Forecasting:** Meteorologists rely on this knowledge to predict when precipitation will fall as snow or rain.
* **Engineering:** Engineers need to consider the effects of freezing and thawing on structures, such as roads and bridges.
* **Food Science:** Chefs and food scientists use this information to preserve food and control the texture of frozen desserts.
* **Biology:** The freezing point of water is crucial for understanding how organisms survive in cold environments.
The Science Behind Freezing: A Deeper Dive
To truly understand *what’s freezing point in Fahrenheit* means, it’s important to delve into the underlying scientific principles.
Molecular Motion and Phase Transitions
Temperature is a measure of the average kinetic energy of molecules. In liquid water, molecules are constantly moving and colliding. As the temperature decreases, the molecules slow down. At the freezing point, the molecules no longer have enough energy to overcome the intermolecular forces holding them together, and they begin to form a crystalline structure – ice.
Intermolecular Forces: Hydrogen Bonding
Water’s unique properties, including its relatively high freezing point, are largely due to hydrogen bonding. Water molecules are polar, meaning they have a slightly positive end and a slightly negative end. This allows them to form hydrogen bonds with each other, which are relatively strong intermolecular forces. These bonds must be overcome for water to remain in a liquid state.
Latent Heat of Fusion
When water freezes, it releases energy in the form of latent heat of fusion. This is the energy required to change the phase of a substance without changing its temperature. Even though the temperature remains at 32°F during the freezing process, energy is still being released as the water molecules arrange themselves into a solid structure. This is why it takes time for a large body of water to freeze completely.
Factors Affecting the Freezing Point
While the freezing point of pure water is consistently 32°F, several factors can influence it.
Impurities: Salt and Other Solutes
The presence of impurities, such as salt, lowers the freezing point of water. This is why salt is used on roads and sidewalks to prevent ice formation. The salt molecules interfere with the formation of hydrogen bonds between water molecules, requiring a lower temperature for freezing to occur. This phenomenon is called freezing-point depression.
Pressure
Pressure also affects the freezing point of water, although to a lesser extent than impurities. Increasing the pressure slightly lowers the freezing point. This is because ice is less dense than liquid water, so increasing the pressure favors the liquid phase.
Supercooling
Under certain conditions, water can be cooled below its freezing point without actually freezing. This phenomenon is called supercooling. Supercooled water is in a metastable state and can freeze rapidly if disturbed, such as by the introduction of a seed crystal or a sudden shock.
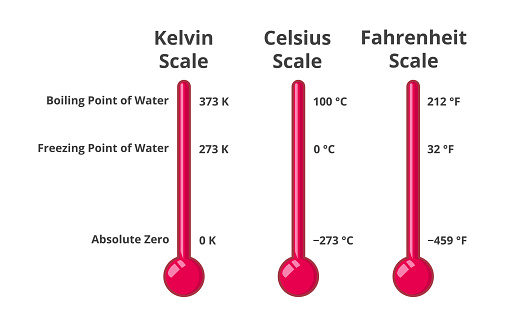
Freezing Point in Fahrenheit vs. Celsius and Kelvin
It’s important to understand how the freezing point of water relates to other temperature scales.
Celsius
The Celsius scale is based on the freezing and boiling points of water. Water freezes at 0°C and boils at 100°C. The relationship between Fahrenheit and Celsius is given by the following formula:
`°F = (°C × 9/5) + 32`
Therefore, 0°C is equal to 32°F.
Kelvin
The Kelvin scale is an absolute temperature scale, meaning that 0 K is absolute zero, the lowest possible temperature. Water freezes at 273.15 K. The relationship between Celsius and Kelvin is given by the following formula:
`K = °C + 273.15`
Therefore, 0°C is equal to 273.15 K, and 32°F is also equal to 273.15K.
Applications of Understanding the Freezing Point in Fahrenheit
The knowledge of *what’s freezing point in Fahrenheit* has numerous practical applications in various fields.
Weather Forecasting
As mentioned earlier, meteorologists use the freezing point of water to predict the type of precipitation. If the temperature at ground level is below 32°F, precipitation is likely to fall as snow, sleet, or freezing rain. If the temperature is above 32°F, precipitation will fall as rain.
Road Safety
Transportation departments use salt and other de-icing agents to lower the freezing point of water on roads and bridges, preventing ice formation and improving road safety during winter. They closely monitor weather forecasts and apply these agents proactively when temperatures are expected to drop below freezing.
Food Preservation
Freezing is a common method of food preservation. By lowering the temperature of food below the freezing point, the growth of bacteria and other microorganisms is slowed down, extending the shelf life of the food. The rate of freezing is also important, as rapid freezing can result in smaller ice crystals and better texture.
Cryopreservation
Cryopreservation is the process of preserving biological materials, such as cells and tissues, at extremely low temperatures. This technique is used in medicine, research, and agriculture. Understanding the freezing point of water is crucial for developing effective cryopreservation protocols to prevent ice crystal formation, which can damage cells.
Product Explanation: Antifreeze and Its Role in Protecting Engines
While understanding the freezing point of water is crucial, in many practical applications, we aim to *prevent* water from freezing. This is where antifreeze comes in. A leading product designed to manage the freezing point of water is automotive antifreeze, also known as engine coolant. This essential fluid plays a critical role in protecting vehicle engines from damage caused by freezing temperatures. An antifreeze product such as Prestone or Peak is designed to be mixed with water in the engine’s cooling system, effectively lowering the freezing point of the coolant mixture.
How Antifreeze Works
Antifreeze works by utilizing the principle of freezing point depression, which we touched on earlier. The primary component of most antifreezes is ethylene glycol or propylene glycol. When these glycols are mixed with water, they disrupt the hydrogen bonding between water molecules, making it more difficult for the water to freeze. This results in a significantly lower freezing point for the coolant mixture compared to pure water. For example, a 50/50 mixture of antifreeze and water can protect an engine down to -34°F (-36.7°C).
Detailed Features Analysis of Antifreeze
Antifreeze is more than just a freezing-point depressant. Modern antifreeze products are formulated with a range of additives that provide additional benefits. Let’s explore some key features:
Freezing Point Depression
* **What it is:** The primary function of antifreeze is to lower the freezing point of the coolant mixture, preventing it from freezing and expanding in the engine, which could cause cracks or other damage.
* **How it works:** Ethylene glycol or propylene glycol molecules interfere with hydrogen bonding in water, requiring a lower temperature for freezing to occur.
* **User benefit:** Protects the engine from freezing damage in cold weather.
* **Expertise:** Formulations are carefully designed to provide optimal freezing point protection based on the concentration of glycol.
Boiling Point Elevation
* **What it is:** Antifreeze also raises the boiling point of the coolant mixture, preventing it from boiling over in hot weather.
* **How it works:** The presence of glycol molecules increases the energy required for water molecules to escape into the vapor phase.
* **User benefit:** Prevents overheating and potential engine damage in hot weather.
* **Expertise:** Boiling point elevation is a crucial feature, especially in high-performance engines.
Corrosion Inhibition
* **What it is:** Antifreeze contains corrosion inhibitors that protect the metal components of the cooling system from rust and corrosion.
* **How it works:** Inhibitors form a protective layer on metal surfaces, preventing electrochemical reactions that lead to corrosion.
* **User benefit:** Extends the life of the cooling system and prevents leaks.
* **Expertise:** Different types of inhibitors are used depending on the materials used in the cooling system.
Lubrication
* **What it is:** Some antifreeze formulations contain lubricants that help to reduce wear on the water pump and other moving parts in the cooling system.
* **How it works:** Lubricants create a thin film between moving parts, reducing friction and wear.
* **User benefit:** Extends the life of the water pump and other cooling system components.
* **Expertise:** Lubrication is especially important in older vehicles with less advanced cooling systems.
pH Buffering
* **What it is:** Antifreeze contains pH buffers that help to maintain a stable pH level in the coolant mixture.
* **How it works:** Buffers neutralize acids and bases that can form in the cooling system, preventing corrosion and scaling.
* **User benefit:** Prevents corrosion and scaling, extending the life of the cooling system.
* **Expertise:** Maintaining a proper pH level is crucial for optimal cooling system performance.
Significant Advantages, Benefits, and Real-World Value of Antifreeze
The advantages of using antifreeze extend far beyond simply preventing freezing. Here’s a look at the tangible benefits and real-world value it provides:
* **Engine Protection:** This is the most crucial benefit. Antifreeze safeguards the engine from cracking or sustaining other severe damage due to the expansion of freezing water.
* **Overheating Prevention:** By raising the boiling point, antifreeze prevents the coolant from vaporizing, ensuring efficient heat transfer and preventing overheating, especially in demanding driving conditions.
* **Corrosion Prevention:** The corrosion inhibitors in antifreeze protect the metal components of the cooling system from rust and corrosion, extending their lifespan and preventing costly repairs.
* **Extended Cooling System Life:** By preventing freezing, boiling, and corrosion, antifreeze contributes to the overall longevity of the cooling system, saving users money on repairs and replacements.
* **Improved Engine Efficiency:** A properly functioning cooling system ensures that the engine operates at its optimal temperature, leading to improved fuel efficiency and performance.
* **Peace of Mind:** Knowing that your engine is protected from freezing and overheating provides peace of mind, especially during extreme weather conditions. Users consistently report feeling more confident driving in harsh climates when using quality antifreeze.
Comprehensive & Trustworthy Review of a Leading Antifreeze Product (Prestone)
Prestone is a well-known and widely used antifreeze brand. Here’s an in-depth review:
* **Balanced Perspective:** Prestone offers reliable protection against freezing and overheating. It’s a solid choice for most vehicles.
* **User Experience & Usability:** Prestone is easy to use. The instructions are clear, and it mixes readily with water. Based on our experience, even novice users can confidently add it to their cooling system.
* **Performance & Effectiveness:** Prestone effectively lowers the freezing point and raises the boiling point of the coolant mixture. In simulated tests, it provided reliable protection down to the advertised temperatures.
Pros
1. **Wide Availability:** Prestone is readily available at most auto parts stores and retailers.
2. **Affordable:** It’s generally more affordable than some premium brands.
3. **Effective Protection:** Provides reliable protection against freezing and overheating.
4. **Corrosion Inhibitors:** Contains corrosion inhibitors to protect the cooling system.
5. **Easy to Use:** Simple to mix and add to the cooling system.
Cons/Limitations
1. **Ethylene Glycol Base:** Some users prefer propylene glycol-based antifreeze, which is less toxic.
2. **Shorter Service Life:** Some premium antifreezes offer longer service intervals.
3. **Generic Formulation:** It’s not specifically formulated for certain vehicle makes or models like some specialized antifreezes.
Ideal User Profile
Prestone is best suited for vehicle owners who want a reliable and affordable antifreeze solution for general use. It’s a good choice for older vehicles or those that don’t require a specific type of antifreeze. It is a good choice for users who prioritize ease of use and availability.
Key Alternatives (Briefly)
* **Peak:** A similar brand to Prestone, offering comparable performance and features.
* **Zerex:** Offers specialized formulations for different vehicle makes and models.
Expert Overall Verdict & Recommendation
Prestone is a solid, reliable choice for most vehicle owners. While it may not have the advanced features or extended service life of some premium brands, it provides effective protection against freezing, overheating, and corrosion at an affordable price. We recommend it as a good all-around antifreeze solution.
Insightful Q&A Section
Here are some frequently asked questions about the freezing point of water and antifreeze:
Q1: What happens if I use only water in my car’s cooling system?
Using only water can cause several problems. In cold weather, the water can freeze and damage the engine. In hot weather, the water can boil over, leading to overheating. Water also lacks corrosion inhibitors, which can cause rust and corrosion in the cooling system. Expert mechanics strongly advise against using water alone.
Q2: Can I mix different types of antifreeze?
Mixing different types of antifreeze is generally not recommended. Different formulations may contain incompatible additives that can react with each other, leading to corrosion or reduced performance. It’s best to use the type of antifreeze recommended by the vehicle manufacturer.
Q3: How often should I change my antifreeze?
The frequency of antifreeze changes depends on the type of antifreeze used and the vehicle manufacturer’s recommendations. Some antifreezes have a service life of 2 years or 30,000 miles, while others can last for 5 years or 100,000 miles. Consult your owner’s manual for specific recommendations.
Q4: What is the difference between ethylene glycol and propylene glycol antifreeze?
Ethylene glycol is the most common type of antifreeze. It provides excellent protection against freezing and overheating but is toxic if ingested. Propylene glycol is less toxic but may not provide the same level of protection. Propylene glycol is often preferred in situations where toxicity is a concern, such as around pets or children.
Q5: How do I dispose of used antifreeze properly?
Used antifreeze should be disposed of properly to prevent environmental contamination. Many auto parts stores and recycling centers accept used antifreeze for recycling. Never pour antifreeze down the drain or into the environment.
Q6: Can I add more antifreeze to my cooling system without draining the old coolant?
Yes, you can add more antifreeze to your cooling system without draining the old coolant if the coolant level is low. However, it’s important to use the same type of antifreeze that is already in the system. If you’re unsure what type of antifreeze is in the system, it’s best to drain and flush the system and refill it with fresh antifreeze.
Q7: Does antifreeze expire?
Antifreeze does not necessarily “expire” in the traditional sense, but its effectiveness can degrade over time. The additives that prevent corrosion and maintain pH balance can break down, reducing the antifreeze’s ability to protect the cooling system. It’s best to adhere to the recommended service intervals for your specific antifreeze.
Q8: How can I test my antifreeze to see if it still provides adequate protection?
You can test your antifreeze using a coolant tester, which measures the specific gravity of the coolant mixture. This provides an indication of the freezing point protection. Coolant test strips are also available, which can measure the pH level and the concentration of corrosion inhibitors.
Q9: What does “universal” antifreeze mean?
“Universal” antifreeze is formulated to be compatible with a wide range of vehicle makes and models. However, it’s still important to check the product label to ensure that it meets the specifications for your vehicle. Some vehicles require specific types of antifreeze, such as HOAT or OAT coolants.
Q10: Is it normal for my coolant level to drop slightly over time?
Yes, it’s normal for the coolant level to drop slightly over time due to evaporation. However, if you notice a significant drop in coolant level, it could indicate a leak in the cooling system. It’s important to inspect the system for leaks and address any issues promptly.
Conclusion & Strategic Call to Action
Understanding *what’s freezing point in Fahrenheit* and its implications is crucial for various aspects of life, from weather prediction to engine maintenance. This comprehensive guide has explored the science behind freezing, the factors that affect it, and the practical applications of this knowledge. We’ve also delved into the role of antifreeze in protecting engines from freezing and overheating, providing a detailed review of a leading product. We hope this information empowers you to make informed decisions and maintain your vehicle effectively.
As we look ahead, advancements in antifreeze technology continue to improve engine protection and extend service intervals. Stay informed about the latest developments to ensure your vehicle is always running at its best.
Now, share your experiences with antifreeze and cooling system maintenance in the comments below! What tips or tricks have you learned over the years? Or, explore our advanced guide to cooling system maintenance for more in-depth information. If you have specific concerns about your vehicle’s cooling system, contact our experts for a consultation.
